Cavitation
- Phenomenon involving the formation and subsequent sudden collapse of vapour cavities in a liquid, with the cavities forming when the local pressure within the flow reduces close to that of the vapour pressure of the liquid.
Understanding cavitation
Cavitation occurs (in siphonic systems) as the formation of vapor bubbles at certain part of the pipeworks due to very low pressure. An example of cavitation can be seen as vapor bubbles forms around a rotating propeller (figure below).
The occurrence of cavitation creates the following problems in siphonic system;
a. Damaging effect to pipe materials
b. Noise
c. Adverse effect on the flow
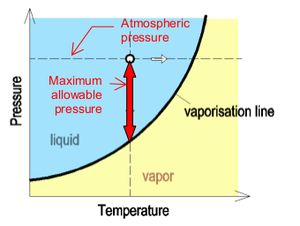
In order to prevent cavitation, the lowest allowable pressure in siphonic systems is controlled to -0.9bar (-90kPa). This value of -0.9bar or -9mH2O is appropriate for installations which are close to mean sea level. Buildings located at high elevations above mean sea level are subjected to lower atmospheric pressure, and the cavitation may occur before negative pressure reach -9mH2O.
Vapor pressure, atmospheric pressure and cavitation
There are 2 conditions where liquid turns into gas (vapor):
i)Increase the temperature while keeping the pressure constant until the temperature is high enough to produce vapor bubbles
ii)Lower the pressure while keeping the temperature constant to a point where the pressure is low enough to produce vapor bubbles
In practical application of siphonic systems, the temperature remains constant.The maximum negative pressure allowed in a siphonic system before cavitation occurs can be understood as the gap between the atmospheric pressure and the vapor pressure of water at a certain temperature.
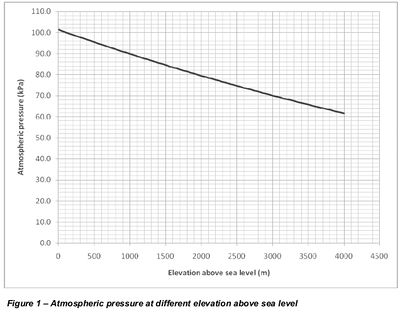
At high altitude, where atmospheric pressure is lower, cavitation occurs at a higher negative pressure than -0.9bar at near sea level.
Air pressure above sea level can be calculated as
p = 101325 (1 - 2.25577 10-5 h)5.25588 where p = air pressure (Pa) h = altitude above sea level (m)
Example:
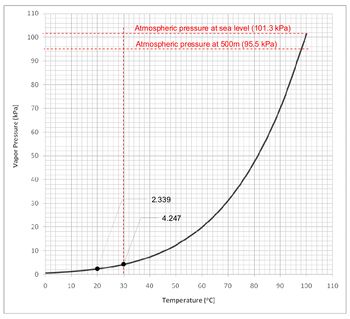
At height of 500m above sea level, the atmospheric pressure is 95.5kPa, assuming the water temperature to be 30oC (worst case scenario) the vapor pressure of water is 4.247kPa. Cavitation will occur at a pressure of:
Vapor pressure – atmospheric pressure
4.247kPa – 95.5kPa = -91.253kPa (-9.3mH2O)
References:
- BS 8490:2007 Guide to siphonic roof drainage systems
- FLUID MECHANICS with Engineering Applications (p.96-98)