Density
A measure of the amount of matter contained by a given volume. Density (ρ) is a physical property found by dividing the mass of an object by its volume. Regardless of the sample size, density is always constant. For example, the density of a pure sample of tungsten is always 19.25 grams per cubic centimetre. This means that whether you have one gram or one kilogram of the sample, the density will never vary. The equation is as follows:
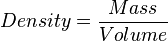
or
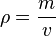
Density can and does vary from element to element and substance to substance due to differences in the relation of mass and volume.
Density and Pressure
Density increases with increasing pressure because volume decreases as pressure increases. And since Density=mass/volume, the lower the volume, the higher the density. The decrease in volume as related to pressure is explained in Boyle's Law: P1V1=P2V2 where P = pressure and V = volume.
Archimedes' Principle
The Greek scientist Archimedes made a significant discovery in 212 B.C. The story goes that Archimedes was asked to find out for the King if his goldsmith was cheating him by replacing his gold for the crown with silver, a cheaper metal. Archimedes did not know how to find the volume of an irregularly shaped object such as the crown, even though he knew he could distinguish between elements by their density. While meditating on this puzzle in a bath, Archimedes recognized that when he entered the bath, the water rose. He then realized that he could use a similar process to determine the density of the crown! He then supposedly ran through the streets naked shouting "Eureka," which means "I found it!" in Latin.
Archimedes then tested the king's crown by taking a genuine gold crown of equal mass and comparing the densities of the two. The king's crown displaced more water than the gold crown of the same mass, meaning that the king's crown had a greater volume and thus had a smaller density than the real gold crown. The king's "gold" crown, therefore, was not made of pure gold. Of course, this tale is disputed today because Archimedes was not precise in all his measurements, which would make it hard to determine accurately the differences between the two crowns.
Archimedes' Principle states that if an object has a greater density than the liquid that it is placed into, it will sink and displace a volume of liquid equal to its own. If it has a smaller density, it will float and displace a mass of liquid equal to its own. If the density is equal, it will not sink or float. This principle also explains why balloons filled with helium float. Balloons, as we learned in the section concerning density and temperature, float because they are less dense than the surrounding air. Helium is less dense than the atmospheric air, so it rises. Archimedes' Principle can also be used to explain why boats float. Boats, including all the air space, within their hulls, are far less dense than water. Boats made of steel can float because they displace their mass in water without submerging all the way.
